BREAKING--Springer Nature Cureus Journal of Medical Science Violates Committee on Publication Ethics (COPE) Guidelines
Publisher Retracts Valid, High Quality, and Widely Read Cureus Paper Calling for Halt in COVID-19 Vaccination
By Peter A. McCullough, MD, MPH
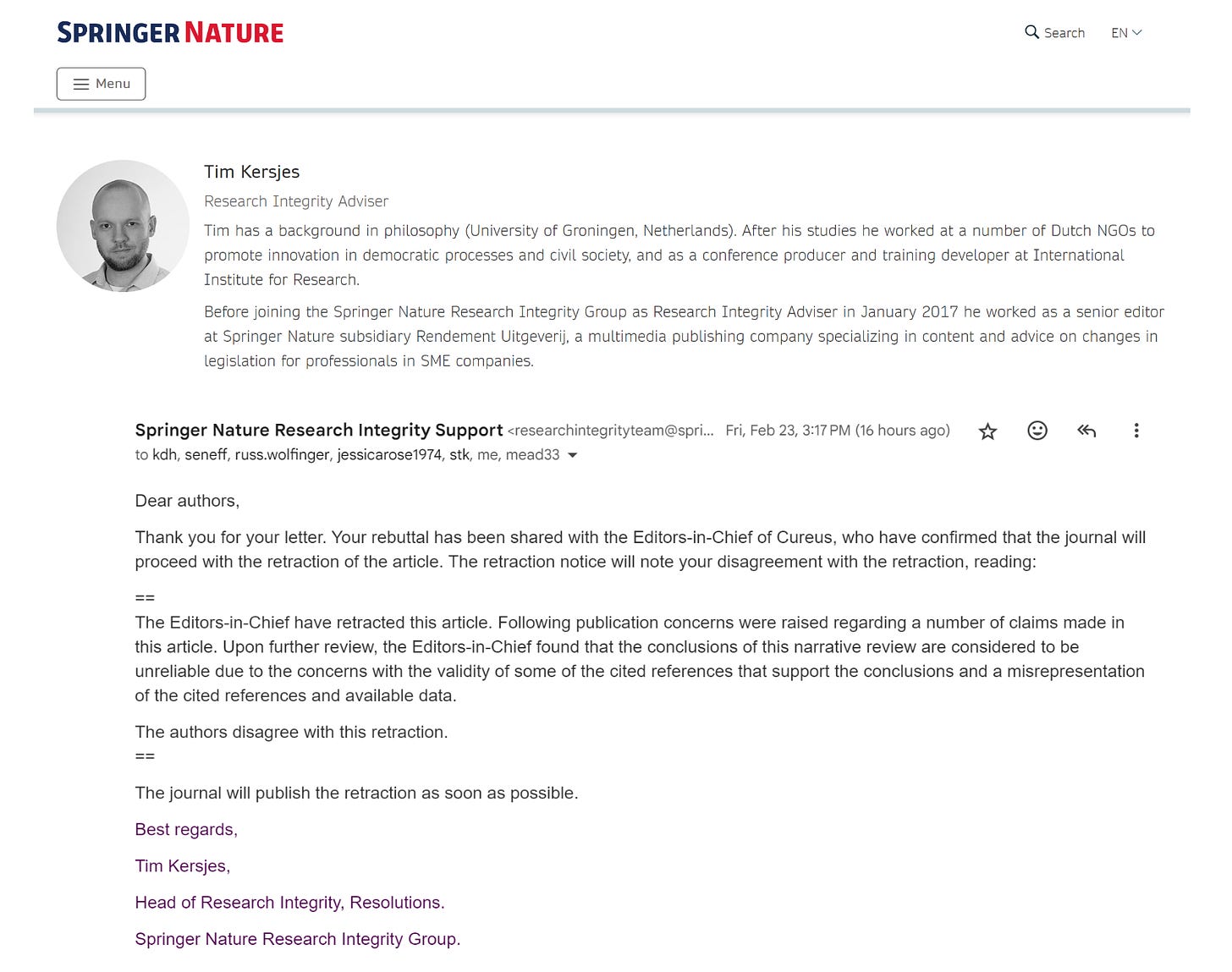
In a stunning act of scientific censorship, a little known publication integrity staffer Tim Kersjes has retracted a manuscript authored by epidemiologist M. Nathaniel Mead, MSc, after the paper drew global attention to the Springer Nature Cureus platform with record views/reads/downloads.
The paper called for a halt in COVID-19 mass vaccination based on a valid evaluation of the evidence. It topped >330,000 views/reads/downloads in a month as compared to an average Cureus-promoted paper which has only ~2700 in a year.
A rating of >9.2 is considered “excellent” and “groundbreaking” appropriately characterizing this extensively cited paper with 293 references (average paper has 30).
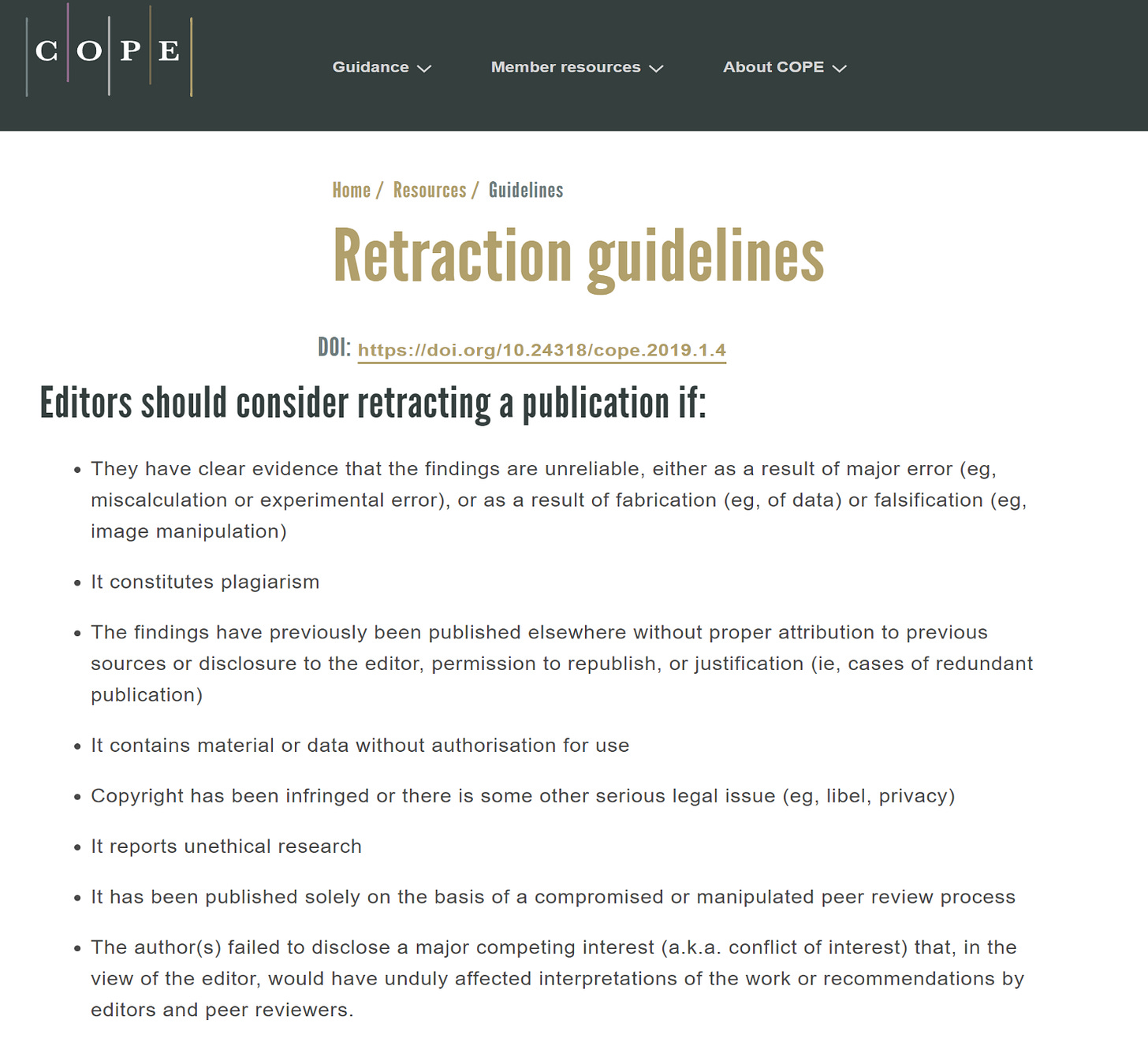
Kersjes raised eight points previously handled in an exhaustive peer-review process. The journal and its editors had the right to reject the paper any time during the review process. Once published, it is a violation of the Committee on Publication Ethics (COPE) Guidelines to retract paper without adequate justification.
The authors’ rebuttal to this bold and unethical action taken by the publisher is posted below so you can see the points raised and responses. Please see the publication link and download your PDF version before it is censored off the platform. Here is the online notice of retraction:
The authors will proceed with publication in an alternate journal as this manuscript garners even more attention in the media because of censorship. See this clip on America’s Voice Live with Steve Gruber February 29, 2024.
Please subscribe to Courageous Discourse as a paying or founder member so we can continue to bring you the truth.
Peter A. McCullough, MD, MPH
President, McCullough Foundation
To: Springer Nature Research Integrity Support
From: M. Nathaniel Mead, BA, BSc, MSc, and Peter A. McCullough, MD, MPH
Subject: RIG-12669 / Concerns regarding our recent article in Cureus
Date: February 22, 2024
To Whom It May Concern:
We are writing in response to the threatened retraction and eight false claims made by Tim Kersjes and Springer Nature regarding our highly rated, heavily viewed and downloaded comprehensive review paper, “COVID-19 mRNA Vaccines: Lessons Learned from the Registrational Trials and Global Vaccination Campaign,” published on 24 January 2024 in the journal Cureus. Mr. Kersjes currently serves as Head of Research Integrity, Resolutions for Springer Nature Research Integrity Group.
The statements made by Kersjes are false, misleading, and unsupported by evidence. Several claims were also arbitrary and capricious. Most of the statements appear to be adapted, either directly or indirectly, from the numerous comments made by the well-known vaccine industry social media trolls, Jonathan Laxton and Matthew Dopler, comments that were inserted almost daily in the Cureus portal following our paper’s publication. Their sole purpose was to deliberately denigrate coauthors of the “Lessons Learned” paper while misleading, confusing, mocking and otherwise upsetting others participating in the Cureus post-publication forum online. As an example, one of Laxton’s statements in the Cureus portal reads as follows:
"This is anti-vaccine gish-gallop that adds nothing useful to the literature. The majority of the authors have no expertise in this subject (Seneff, Rose, Kirsch). They wildly speculate on "anomalies" in the Pfizer trial even though data from the Moderna trial is similar with similar efficacy outcomes and the Clalit real-world study (Pfizer-funded) found a similar efficacy rate. They misrepresent all-cause mortality as COVID-19 would have to make up a very large amount of deaths to detect a difference in all-cause mortality between groups in a study of this size. They misrepresent VAERs data ignoring the number of doses given at once for COVID-19 and that the COVID vaccine is 2-3 doses and influenza 1. They present the Table 2 fallacy from the Cleveland Clinic study. This study never should have been published." - Jonathan Laxton, MD
This statement from Laxton is nonsense from a scientific standpoint. Following the libelous, defamatory comments about several of our paper’s coauthors, Laxton resorts to incoherent rambling and gibberish about the Pfizer and Moderna trials, followed by his citing a non-existent “Table 2 fallacy”; both comments may suggest an intellectual disability or some form of cognitive impairment. We have placed in bold the phrases that Kersjes clearly appropriated from Laxton in order to help devise the first two of eight statements in the retraction letter. Kersjes evidently borrowed these phrases without realizing that (a) his actions would be uncovered and exposed, and (b) neither Laxton nor Dopler have any training in epidemiology and biostatistics, hence the outright speciousness of their comments. Unfortunately for Kersjes, the groundless, misleading comments of both Laxton and Dopler contribute nothing of substance and instead only engender confusion and cognitive dissonance for the Cureus readership. Given that Kersjes has no training in the health sciences, it is not surprising that he fails to see through the inaccurate and fallacious nature of the numerous Laxton/Dopler comments.
In sum, the false claims made by Kersjes reflect a breathtaking dearth of scientific understanding. All of Kersjes’s comments were accounted for and subsumed by the review process; more sophisticated and meaningful variations of these comments were raised by the original reviewers of the manuscript and were handled with edits in the paper during the review process. The Cureus editors found the responses satisfactory and fully accepted the paper. The manuscript has been contracted and copyrighted, and has had a record number of views, reads, and downloads over a one month period for Cureus. Nevertheless, we will now respond to each of Kersjes’s statements in turn.
1) Kersjes claim: We find that the article is misrepresenting all-cause mortality data
Response: In borrowing this comment from Laxton, Kersjes is offering an unfounded criticism. Three sources of ACM data were considered in our paper, and we accurately synopsized all three: the Benn et al. (2022), Aarstad and Kvitastein (2022), and Rancourt et al. (2023) analyses. The paper’s eight reviewers’ agreed with the way we presented these ACM data. All agreed that the Benn et al. analysis showed the mRNA vaccinations did not lead to a reduction in overall mortality. All agreed that the Aarstad and Kvitastein analysis of 31 European countries showed “(a) increases in ACM during the initial nine-month period of 2022 were positively correlated with increases in 2021 vaccination distribution; and (b) each percentage point increase in 2021 vaccination coverage was associated with a 0.105% increase (95%CI 0.075-0.134) in monthly mortality during 2022.” Reviewer Alpha (evidently chosen by Cureus) asked us to emphasize the Rancourt et al. 180-page analysis extensively showed booster rollouts synchronously followed by peaks in all-cause mortality, so we inserted that important statement as well.
Citations
#52. Benn CS, Schaltz-Buchholzer F, Nielsen S, et al.: Randomised clinical trials of COVID-19 vaccines: do adenovirus-vector vaccines have beneficial non-specific effects?. Lancet preprint. April. 5:2022. 10.2139/ssrn.4072489
#254. Aarstad J, Kvitastein OA: Is there a link between the 2021 COVID-19 vaccination uptake in Europe and 2022 excess all-cause mortality?. Asian Pac J Health Sci. 2022, 2023:25-31. 10.21276/apjhs.2023.10.1.6
#255. Rancourt DG, Baudin M, Hickey J, Mercier J: COVID-19 Vaccine-Associated Mortality in the Southern Hemisphere. Correlation Research in the Public Interest, Ontario, Canada; 2023.
Our reanalysis of the postmarketing data provided to the FDA for the Pfizer trial data yielded a 31% higher ACM risk in the BNT162b2 group compared to the placebo group. The detailed explanation for this calculation can be found in the second to last paragraph in the section, “Revisiting the registrational trials”. This was thoroughly analyzed by all eight reviewers, with no objections. We clearly noted that the 31% increase was not a statistically significant finding, but still a trend in the wrong direction. The 31% increase and theoretical estimate of the timeframe that would be required to reach statistical significance (2.8 years) was independently confirmed by Masterjohn (citation #64).
2) Kersjes claim: We find that the article appears to be misrepresenting VAERs data
Response: Kersjes is making an oblique, unsubstantiated comment, again borrowing directly from Laxton’s comment above. Our two charts were based on documented queries to the Vaccine Adverse Events Reporting System (VAERS). There is no misrepresentation because the charts were generated directly from data supplied by these VAERS queries. In the last paragraph in the section “Quality control issues and process-related impurities”, our paper states the following: “Based on a query of the MedDRA code ‘Autoimmune disorder’ in the Vaccine Adverse Events Reporting System (VAERS), there was an 803% increase in autoimmune disorders per million doses administered when comparing the administration of Influenza vaccines from 2018 to 2020 with COVID-19 vaccinations from 2021 to 2023 (Figure 5) [173]. This represents an immense safety signal.” All eight reviewers agreed with this wording and interpretation.
In the second paragraph of the Discussion, we offer this clear caveat about the use of VAERS: “Although invaluable as tools for detecting safety signals, national health surveillance databases such as VAERS and Yellow Card do not meet the rigorous standards set by controlled trials, further underscoring the necessity of this approach for the assessment of medical and public health interventions.” This is why we only cite VAERS as indicative of safety signals and concerning trends.
Referring to Figure 7 in the Discussion, we offered the following interpretation and substantiation: “Figure 7 shows a graph based on myocarditis reports in VAERS Domestic Data as of September 29, 2023, which offers an indication of the gravity of this situation. All myocarditis reports are plotted according to age and dose (dose 1 (pink), dose 2 (green), and dose 3 (blue)). After dose two, there was a five-fold increase in myocarditis cases among 15-year-old males. Regardless of age, myocarditis cases were more frequent following dose two, which is suggestive of a causal link between myocarditis and the COVID-19 mRNA inoculations. The data depicted in the chart are further reinforced by a recent disproportionality analysis of VAERS data showing a statistically significant association between cardiovascular events and COVID-19 vaccinations [263].”
All eight reviewers agreed with this concise, cogent presentation of the VAERS data. Note that we use the phrase “suggestive of a causal link”. We are not declaring a cause-and-effect relationship, only suggesting the possibility on a precautionary basis. The most compelling study of VAERS is the large disproportionality analysis by Yan et al.(2022), which is citation #48 in our paper. This study found very strong signals for myocardial infarction, pulmonary embolism, cardio-respiratory arrest, cerebral infarction, ischemic stroke, and cerebral hemorrhage associated with both mRNA vaccines.
Citations
#48. Yan MM, Zhao H, Li ZR, et al.: Serious adverse reaction associated with the COVID-19 vaccines of BNT162b2, Ad26.COV2.S, and mRNA-1273: gaining insight through the VAERS. Front Pharmacol. 2022, 13:921760. 10.3389/fphar.2022.921760
#173. VAERS Reports Contradict Claim of No AEs in Frameshifting Context. (2023). Accessed: December 16, 2023: https://jessicar.substack.com/p/vaers-reports-contradict-claim-of.
#263. Amir M, Latha S, Sharma R, Kumar A: Association of cardiovascular events with COVID-19 vaccines using vaccine adverse event reporting system (VAERS): a retrospective study. Curr Drug Saf. 2023, 10.2174/0115748863276904231108095255
3) Kersjes claim: The article states that the Pfizer COVID-19 vaccine saved two lives and caused 27 deaths per 100,000 vaccinations, and the Moderna vaccine saved 3.9 lives and caused 10.8 deaths per 100,000 vaccinations, though there does not appear to be convincing evidence for this claim.
Response: Kersjes is referring to Appendix 2, but he fails to understand that this is not a matter of evidence but biostatistical reasoning and calculation. The calculations were derived and presented as estimates based on conservative assumptions that are clearly explained and delineated in Appendix 2. The fact that Kersjes points to not enough “convincing evidence” to support this estimate suggests a fundamental lack of understanding of epidemiological principles, methods and procedures, much less how to place quantitative analyses in context. The calculation of number of lives saved per 100K vaccinations was in fact based on generous assumptions of benefit, utilizing data from the relatively healthy population recruited for the Pfizer trial. It was also based on conservative assumptions of risk based on the Fenton analysis of UK Yellow Card data.
The epidemiological and biostatistical rationale for Appendix 2 is that any risk-benefit analysis must consider how much the presumed benefit in terms of reducing COVID-19-related mortality is offset by the potential increase in vaccine-induced mortality. Assessments of the safety profile of the Covid-19 modified mRNA injections warrant an objective, precautionary perspective. Any substantial upward trend in all-cause mortality within the intervention arm of the study population reflects poorly on the intervention and indicates an unacceptable safety risk to the general public. All eight reviewers agreed with this rationale and several applauded us for using it because they understood that, from a precautionary standpoint, our analytical approach was balanced and appropriate.
4) Kersjes claim: Incorrect claim: Vaccines are gene therapy products.
Response: Here Kersjes is making a simplistic and distorted interpretation of what we stated in our paper. The focus of the paragraph in question (see below) was on the terminology that has been in use for the 30 years preceding the pandemic. During that time, as we state in the paper, the terms gene therapy and mRNA vaccination were often used interchangeably [23]. The third to last paragraph in the Introduction reads as follows:
Concerns about inadequate safety testing extend beyond the usual regulatory approval standards and practices. Although we employ the terms “vaccine” and “vaccination” throughout this paper, the COVID-19 mRNA products are also accurately termed gene therapy products (GTPs) because, in essence, this was a case of GTP technology being applied to vaccination [21]. European regulations mandate the inclusion of an antigen in vaccines, but these immunogenic proteins are not intrinsic to the mRNA vaccines [22]. The GTP vaccine platform has been studied for over 30 years as an experimental cancer treatment, with the terms gene therapy and mRNA vaccination often used interchangeably [23]. This is due to the mRNA products’ specific mode of action: synthetic mRNA strands, encapsulated within a protective lipid nanoparticle (LNP) vehicle, are translated within the cells into a specific protein that subsequently stimulates the immune system against a specific disease. Another accurate label would be prodrugs because these products stimulate the recipient’s body to manufacture the target protein [24]. As there were no specific regulations at the time of the rapid approval process, regulatory agencies quickly “adapted” the products, generalized the definition of “vaccine” to accommodate them, and then authorized them for EUA for the first time ever against a viral disease. However, the rationale for regulating these products as vaccines and excluding them from regulatory oversight as GTPs lacks both scientific and ethical justification [21]. (Note: Throughout this review, the terms vaccines and vaccinations will be used interchangeably with injections, inoculations, biologicals, or simply, products.)
All eight reviewers agreed with the content of the above paragraph, as did the Cureus editors.
Citations:
#21. Banoun H: mRNA: vaccine or gene therapy? he safety regulatory issues. Int J Mol Sci. 2023, 24:10514. 10.3390/ijms241310514
#22. Guerriaud M, Kohli E: RNA-based drugs and regulation: toward a necessary evolution of the definitions issued from the European Union legislation. Front Med (Lausanne). 2022, 9:1012497. 10.3389/fmed.2022.1012497
#23. Van Lint S, Renmans D, Broos K, et al.: The ReNAissanCe of mRNA-based cancer therapy. Expert Rev Vaccines. 2015, 14:235-51. 10.1586/14760584.2015.957685
#24. Cosentino M, Marino F: Understanding the pharmacology of COVID-19 mRNA vaccines: playing dice with the spike?. Int J Mol Sci. 2022, 23:10881. 10.3390/ijms231810881
5) Kersjes claim: The article states that vaccines are contaminated with high levels of DNA. Upon review we found that the cited references are not sufficient to support these claims.
Response: This is a mere hand-waving argument. The references were sufficient as we will now show. Yu et al. were the first to report the issue of batch variability [150]. Speicher et al. then reported DNA fragments detected in monovalent and bivalent Pfizer/BioNTech and Moderna modRNA COVID-19 vaccines from Ontario, Canada [151]. In an analysis of multiple vials of the bivalent Pfizer and Moderna mRNA products, McKernan et al. found “high levels of DNA contamination in both the monovalent and bivalent vaccines” that were “orders of magnitude higher than the EMA's limit” of 330 nanograms of DNA per milligram of RNA [152]. The DNA process-related impurities also exceeded the safety limits of the FDA (10ng/dose). These data were then independently confirmed by Buckhaults and his genomics research team. They examined two batches of Pfizer mRNA vials and confirmed contamination with the plasmid DNA vector that had been used as the template for mRNA vaccine production [8,153]. At a South Carolina Senate hearing, Buckhaults reported having consistently sequenced substantial quantities of plasmid DNA, 200 billion DNA fragments per vial [153].
We acknowledge that the research on process-related impurities (bacterial DNA fragments) is quite new and therefore controversial. Our discussion of process-related impurities was the very last section included in this narrative review, and it was inserted at the behest of reviewer alpha (again, ostensibly chosen by Cureus) in order to better address the manufacturing and quality control issues. All eight reviewers agreed that this was a serious issue and that, contrary to your assertion, the citations provided were sufficient. We understand that this is not an issue the vaccine enterprise wants to be made public, and it is alarming to see Springer Nature, the largest medical publisher in the world, evidently attempting to censor such information. DNA contamination has emerged as perhaps the most dangerous aspect of these experimental products because of the potential threat of integration into the human genome. Again, the precautionary principle should be honored if protection of public health is to be considered paramount.
Citations:
#150. Yu B, Taraban MB, Briggs KT: All vials are not the same: potential role of vaccine quality in vaccine adverse reactions. Vaccine. 2021, 39:6565-9. 10.1016/j.vaccine.2021.09.065
#151. Speicher DJ, Rose J, Gutschi, Wiseman DM, McKernan K: DNA fragments detected in monovalent and bivalent Pfizer/BioNTech and Moderna modRNA COVID-19 vaccines from Ontario, Canada: exploratory dose response relationship with serious adverse events [PREPRINT]. OSFPreprints. 2023, 10.31219/osf.io/mjc97
#152. McKernan K, Helbert Y, Kane LT, McLaughlin S: Sequencing of bivalent Moderna and Pfizer mRNA vaccines reveals nanogram to microgram quantities of expression vector dsDNA per dose [PREPRINT]. OSFPreprints. 2023, 10.31219/osf.io/b9t7m
#153. Senate H∂earing On Dangerous and Potentially Fatal Errors Within The Methods of Vaccine Distribution.. ( September 20, 2023.). Accessed: January 17, 2023: https://arvozylo.medium.com/senate-hearing-on-dangerous-and-potentially-fatal-errors-within-the-methods-of-vaccine-di....
6) Kersjes claim: The article states that SV40 promoter can cause cancer because SV40 virus can cause cancer in some organisms and inconclusively in humans. However, we find that this is misrepresenting the cited study (Li, S., MacLaughlin, F., Fewell, J. et al. Muscle-specific enhancement of gene expression by incorporation of SV40 enhancer in the expression plasmid. Gene Ther 8, 494–497 (2001). https://doi.org/10.1038/sj.gt.3301419
Response: Ironically, this statement from Kersjes is, in fact, misrepresenting our content. For citation 159, we stated the following: “In a 2001 study on somatic gene delivery to skeletal muscle cells, it was shown that incorporation of the SV40 enhancer into DNA plasmids could increase the level of exogenous gene expression by a factor of 20 [159].” The statement is perfectly accurate, and the Cureus editors and reviewers agreed with this interpretation. We are sorry if Kersjes has once again humiliated himself and his corporate superiors by exploiting or consulting with Dopler and/or Laxton to make this erroneous claim.
Additionally, we cite several studies to support the potential causal connection between SV40 and cancer [155-158], again entirely relevant when considering the precautionary principle. Moreover, concerns around the SV40 sequence being present in the bacterial DNA were also cited in a 2023 Cureus article by Angues and Bustos, a paper focusing on the multi-hit hypothesis of oncogenesis.
Citations
#155.Vilchez RA, Butel JS: Emergent human pathogen simian virus 40 and its role in cancer. Clin Microbiol Rev. 2004, 17:495-508. 10.1128/CMR.17.3.495-508.2004
#156. Rotondo JC, Mazzoni E, Bononi I, Tognon M, Martini F: Association between simian virus 40 and human tumors. Front Oncol. 2019, 9:670. 10.3389/fonc.2019.00670
#157. Vilchez RA, Kozinetz CA, Arrington AS, et al.: Simian virus 40 in human cancers. Am J Med. 2003, 114:675-84. 10.1016/s0002-9343(03)00087-1
#158. Qi F, Carbone M, Yang H, Gaudino G: Simian virus 40 transformation, malignant mesothelioma and brain tumors. Expert Rev Respir Med. 2011, 5:683-97. 10.1586/ers.11.51
#159. Li S, MacLaughlin FC, Fewell JG, et al.: Muscle-specific enhancement of gene expression by incorporation of SV40 enhancer in the expression plasmid. Gene Ther. 2001, 8:494-7. 10.1038/sj.gt.3301419
#160. Orient JM: Beyond negative evidence: Lessons from the disputes on DNA contamination of COVID-19 vaccines. J Am Phys Surg. 2023, 28:106-12.
#219. Angues VR, Bustos PY: SARS-CoV-2 vaccination and the multi-hit hypothesis of oncogenesis. Cureus. 2023, 15:e50703. 10.7759/cureus.50703
7) Kersjes claim: The article states that mRNA COVID-19 vaccines did not undergo adequate safety and efficacy testing, which the journal considers to be incorrect.
Response: Once again, Kersjes provides no evidence to support this claim. This statement sounds like a broad-sweeping position statement being issued by the publisher and by the vaccine industry stakeholders, certainly not from the journal itself. We worked closely with the journal’s editors and they never once made such a comment, because they could see that the evidence and arguments we presented were sound.
First, the serious adverse events, or SAEs, are all well-documented in our paper. Second, the registrational trials were far too short to able to assess safety (2-3 months, in contrast with the previous industry standard of 10-15 years). Third, the trials underreported and underestimated the SAEs, and these underestimations were only demonstrated later on (see Fraiman et al. and Michels et al. re-analyses). In order to help Kersjes see the error of his thinking, we recommend that he sift through and study all our paper’s 294 scientific citations in order to more clearly understand the serious nature of the situation. Only by studying these reports and analyses can you honestly come to recognize and comprehend the profoundly unsafe nature of these mRNA products. As the Dutch saying goes, “van fouten kun je leren”—you can learn from your mistakes.
Examples can be found throughout our paper. Based on re-analyses of the data from Pfizer’s founding trial, Michels and colleagues found a nearly four-fold increase (OR 3.7, 95%CI 1.02-13.2, p = 0.03) in serious cardiac events (e.g., heart attack, acute coronary syndrome) in the vaccine group. Neither the original trial report nor Pfizer’s Summary Clinical Safety report acknowledged or commented on this crucial safety signal. Moreover, the Fraiman et al. re-analysis of the Pfizer trial found a significant 36% higher risk of serious adverse events (SAEs), which included deaths and many life-threatening conditions in the vaccinated participants [50]. Fraiman’s group also found a four-fold higher risk of serious adverse events of special interest (AESIs) compared to the risk of COVID-19 hospitalizations, while the Moderna trial demonstrated a more than 2-fold higher risk. For perspective, the official SAE rate for other vaccines is only 1-2 per million. The Fraiman team’s estimate based on the Pfizer trial data (1,250 SAEs per million) exceeds this benchmark by at least 600-fold. An independent risk-benefit analysis by Mörl et al. 2022 [51] showed that the Pfizer mRNA vaccine produced 25 times more SAEs than the number of severe COVID-19 cases prevented. As reported by Montano in 2021 [47], two large drug safety reporting systems in the US and Europe found 7.8 million vaccine-related adverse events among approximately 1.6 million individuals, with older age groups showing a higher frequency of death, hospitalizations, and life-threatening reactions. Signals were identified for heart attack, pulmonary embolism, cardio-respiratory arrest, cerebral infarction, and cerebral hemorrhage, with relative risk estimates ranging from approximately 1.5 to 8.5.
In summary, this idea that the “mRNA COVID-19 vaccines did not undergo adequate safety and efficacy testing” is the central premise of our entire paper. It was the basis for our paper being considered by Cureus in the first place, and then eventually accepted following an exhaustive, lengthy review process. The fact that Kersjes is making this statement suggests that he and his Springer superiors are overriding the Cureus editors in this matter (and scapegoating the editors in the process). After extensive back-and-forth communications, revisions, and follow-up confirmation, the Cureus reviewers and editors concurred with our conclusions, which resulted in acceptance of the paper for publication.
The following citations from our paper provide a sampling of studies, analyses, and evidence-based reviews that help drive home the seriously unsafe nature of these COVID-19 mRNA products:
#47. Montano D: Frequency and associations of adverse reactions of COVID-19 vaccines reported to pharmacovigilance systems in the European Union and the United States. Front Public Health. 2021, 9:756633. 10.3389/fpubh.2021.756633
#48. Yan MM, Zhao H, Li ZR, et al.: Serious adverse reaction associated with the COVID-19 vaccines of BNT162b2, Ad26.COV2.S, and mRNA-1273: gaining insight through the VAERS. Front Pharmacol. 2022, 13:921760. 10.3389/fphar.2022.921760
#49. Classen B: US COVID-19 vaccines proven to cause more harm than good based on pivotal clinical trial data analyzed using the proper scientific endpoint, “all cause severe morbidity”. Trends Int Med. 2021, 1:1-6.
#50. Fraiman J, Erviti J, Jones M, Greenland S, Whelan P, Kaplan RM, Doshi P: Serious adverse events of special interest following mRNA COVID-19 vaccination in randomized trials in adults. Vaccine. 2022, 40:5798-805. 10.1016/j.vaccine.2022.08.036
#51. Mörl F, Günther M, Rockenfeller R: Is the harm-to-benefit ratio a key criterion in vaccine approval?. Front Med (Lausanne). 2022, 9:879120. 10.3389/fmed.2022.879120
#54. Michels CA, Perrier D, Kunadhasan J, et al.: Forensic analysis of the 38 subject deaths in the 6- month interim report of the Pfizer/BioNTech BNT162b2 mRNA vaccine clinical trial. IJVTPR. 2023, 3:973-1009. 10.56098/ijvtpr.v3i1.85
#57. Oster ME, Shay DK, Su JR, et al.: Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA. 2022, 327:331-40. 10.1001/jama.2021.24110
#58. Rees AR: Viruses, vaccines and cardiovascular effects. Br J Cardiol. 2022, 29:16. 10.5837/bjc.2022.016
#59. Almas T, Rehman S, Mansour E, et al.: Epidemiology, clinical ramifications, and cellular pathogenesis of COVID-19 mRNA-vaccination-induced adverse cardiovascular outcomes: a state-of-the-heart review. Biomed Pharmacother. 2022, 149:112843. 10.1016/j.biopha.2022.112843
#60. Gao J, Feng L, Li Y, et al.: A systematic review and meta-analysis of the association between SARS-CoV-2 vaccination and myocarditis or pericarditis. Am J Prev Med. 2023, 64:275-84. 10.1016/j.amepre.2022.09.002
#61. Yasmin F, Najeeb H, Naeem U, et al.: Adverse events following COVID-19 mRNA vaccines: a systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun Inflamm Dis. 2023, 11:e807. 10.1002/iid3.807
#62. Shiravi AA, Ardekani A, Sheikhbahaei E, Heshmat-Ghahdarijani K: Cardiovascular complications of SARS-CoV-2 vaccines: an overview. Cardiol Ther. 2022, 11:13-21. 10.1007/s40119-021-00248-0
#63. Jeet Kaur R, Dutta S, Charan J, et al.: Cardiovascular adverse events reported from COVID-19 vaccines: a study based on WHO database. Int J Gen Med. 2021, 14:3909-27. 10.2147/IJGM.S324349
#71. Gøtzsche PC, Demasi M: Serious harms of the COVID-19 vaccines: a systematic review [PREPRINT]. medRxiv. 2022, 10.1101/2022.12.06.22283145
#139. Hulscher N, Alexander PE, Amerling R, et al.: A systematic review of autopsy findings in deaths after COVID-19 vaccinations. Zenodo. 2023, 10.5281/zenodo.8120770
#140. Hulscher N, Hodkinson R, Makis W, McCullough PA: Autopsy findings in cases of fatal COVID-19 vaccine-induced myocarditis. ESC Heart Failure. 2024, 1-14. 10.1002/ehf2.14680
#141. Schwab C, Domke LM, Hartmann L, et al.: Autopsy-based histopathological characterization of myocarditis after anti-SARS-CoV-2-vaccination. Clin Res Cardiol. 2023, 112:431-440. 10.1007/s00392-022-02129-5
#142. Pathology Conference: Vaccine-induced spike protein production in the brain, organs etc., now proven [Webpage in German]. (2022). Accessed: October 16, 2023: https://report24.news/pathologie-konferenz-impfinduzierte-spike-produktion-in-gehirn-u-a-organen-nun-erwiesen/.
#143. Reutlingen Autopsy/Histology Study: Side-effects from corona vaccinations [Webpage in German]. (2020). Accessed: October 16, 2023: https://corona-blog.net/2022/03/10/reutlinger-autopsie-histologie-studie-nebenwirkungen-und-todesfaelle-durch-die-cor....
#144. Seneff S, Kyriakopoulos AM, Nigh G, McCullough PA: A potential role of the spike protein in neurodegenerative diseases: a narrative review. Cureus. 2023, 15:e34872. 10.7759/cureus.34872
#207. Liu J, Wang J, Xu J, et al.: Comprehensive investigations revealed consistent pathophysiological alterations after vaccination with COVID-19 vaccines. Cell Discov. 2021, 7:99. 10.1038/s41421-021-00329-3
#208. Collier JL, Weiss SA, Pauken KE, Sen DR, Sharpe AH: Not-so-opposite ends of the spectrum: CD8(+) T cell dysfunction across chronic infection, cancer and autoimmunity. Nat Immunol. 2021, 22:809-19. 10.1038/s41590-021-00949-7
#209. Irrgang P, Gerling J, Kocher K, et al.: Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci Immunol. 2023, 8:eade2798. 10.1126/sciimmunol.ade2798
#210. Uversky VN, Redwan EM, Makis W, Rubio-Casillas A: IgG4 antibodies induced by repeated vaccination may generate immune tolerance to the SARS-CoV-2 spike protein. Vaccines (Basel). 2023, 11:99. 10.3390/vaccines11050991
8) Kersjes claim: The article incorrectly states that spike proteins produced by COVID-19 vaccination linger in the body and cause adverse effects.
Response: Kersjes makes this false claim without citing any evidence. In our review, we cite numerous studies that contradict his claim regarding spike protein-induced adverse effects. For example, upon biopsy in young individuals hospitalized with COVID-19 vaccine myocarditis, Baumeier et al. found spike protein (s-protein) in the heart associated with the active inflammation of myocarditis. Several studies cited below found spike protein causing inflammation and damage in the heart, brain and other organs. Yonker et al. [202] observed that the S-protein persists in circulation in young adults who developed myocarditis post mRNA vaccination, but not in vaccinated individuals who did not develop myocarditis.
As Parry et al. [180] document in great detail, the modified nature of the synthetic mRNA (pseudouridine mRNA) accounts for the prolonged mRNA persistence and spike protein production throughout the body. Röltgen et al. [236] found persistence of both vaccine mRNA and spike protein for the full 60 days duration of their study; the mRNA and free spike proteins were found in the cytoplasm and nuclei of germinal cells in axillary lymph nodes ipsilateral to deltoid muscle injection site. The persistence of these mRNA vaccine components is due to the fact that the synthetic mRNA is excessively stable over the course of months, while “natural” mRNA degrades rapidly. Trougakos et al. [25] surmise that “vaccination-mediated adverse effects (AEs) can be attributed to the unique characteristics of the S-protein itself (antigen) either due to molecular mimicry with human proteins or as an ACE2 ligand.”
Again, Kersjes provides no evidence to support his claim and instead resorts to arbitrary hand-waving statements. We encourage him and his handlers at Springer to stop denying what the scientific evidence is showing to be the foundational basis for the SAEs that have been clearly demonstrated to be either caused or strongly associated with these experimental genetic injections.
Citations
24. Cosentino M, Marino F: Understanding the pharmacology of COVID-19 mRNA vaccines: playing dice with the spike?. Int J Mol Sci. 2022, 23:10881. 10.3390/ijms231810881
25. Trougakos IP, Terpos E, Alexopoulos H, et al.: Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. 2022, 28:542-54. 10.1016/j.molmed.2022.04.007
142. Pathology Conference: Vaccine-induced spike protein production in the brain, organs etc., now proven [Webpage in German]. (2022). Accessed: October 16, 2023: https://report24.news/pathologie-konferenz-impfinduzierte-spike-produktion-in-gehirn-u-a-organen-nun-erwiesen/.
175. Bellavite P, Ferraresi A, Isidoro C: Immune response and molecular mechanisms of cardiovascular adverse effects of spike proteins from SARS-CoV-2 and mRNA vaccines. Biomed. 2023, 11:451. 10.3390/biomedicines11020451
180. Parry PI, Lefringhausen A, Turni C, Neil CJ, Cosford R, Hudson NJ, Gillespie J: ’Spikeopathy’: COVID-19 spike protein is pathogenic, from both virus and vaccine mRNA. Biomed. 2023, 11:2287. 10.3390/biomedicines11082287
202. Yonker LM, Swank Z, Bartsch YC, et al.: Circulating spike protein detected in post- COVID-19 mRNA vaccine myocarditis. Circulation. 2023, 147:867-76. 10.1161/CIRCULATIONAHA.122.061025
203. Baumeier C, Aleshcheva G, Harms D, et al.: Intramyocardial inflammation after COVID-19 vaccination: an endomyocardial biopsy-proven case series. Int J Mol Sci. 2022, 23:6940. 10.3390/ijms23136940
Mr. Kersjes alludes to the journal’s Editors-in-Chief as the source of these publication concerns. This appears to be a duplicitous act of scapegoating. The assertion is wholly implausible, as we worked closely with the Cureus editors for several months, and they ultimately approved the paper for publication, following an intensive review process that lasted several months and included multiple editors and reviewers. Indeed, all of the above issues were previously raised and successfully addressed by the Cureus editors and reviewers, resulting in the final acceptance and publication of the paper after completing the peer review process.
Careful inspection of the nature and manner of presenting these eight points for retraction makes it quite clear that Kersjes overrode the Cureus editors in criticizing the validity of our paper. Instead, he and his Springer superiors rely mainly on the unsubstantiated, misleading comments of the vaccine industry trolls, Laxton and Dopler. This is somewhat understandable, since Kersjes has no first-authored, peer-reviewed publications and no high-level scientific training, thus no capacity to judge or opine on the validity of the paper. He therefore cannot be considered a content expert on COVID-19 vaccine safety, and his questions indicate that he never once consulted with experts on COVID-19 vaccination. In short, Kersjes is unqualified to make an ex post facto decision on publication validity. The eight point list appears to be his personal attempt to scrub the permanent record on vaccine safety. Nevertheless, in offering the eight statements, Kersjes has violated the COPE guidelines. Any final decision regarding the retraction of the paper will have to be taken out of his hands since he is on the COPE committee, presenting a clear conflict of interest.
We vigorously reject this opinionated, ex post facto, arbitrary, and capricious decision on the part of Kersjes and his Springer superiors. If this article is retracted, we will report Springer Nature and the Cureus editors (whose integrity we believe is being unfairly impugned in this matter) to be in violation of the COPE guidelines. We will then bring this matter to the attention of the Committee on Publication Ethics. Furthermore, we will contact the editorial board of Cureus to express our concerns, as we do not believe they had predatory intentions (i.e., deliberately accepting the paper in order to then retract it in a devious attempt to undermine its validity and credibility). Finally, we may also appeal to the U.S. Office of Research Integrity (ORI), which is part of the U.S. Department of Health and Human Services.
In closing, we note that the average Cureus-promoted paper receives 2700 views in a year. Our “Lessons Learned” paper has received more than 330,000 views in a single month. Our Scholarly Impact Quotient (SIQ) rating is just under 9.3, therefore outstanding and indeed unprecedented, given that no previous Cureus paper has elicited this much attention, interest and enthusiasm in such a short period of time. We challenge the publisher to show us evidence to the contrary. If this paper is retracted, it will be in violation of the COPE Guidelines resulting in both the Journal, Kersjes, and Springer being reported to multiple organizations for violation of publication ethics, integrity, and breach of contract.
All of the authors of THIS paper reject this threatened retraction.
Sincerely,
M. Nathaniel Mead MSc, lead author
Peter A. McCullough MD, MPH, senior author (694 listings in PUBMED)
Stephanie Seneff PhD
Russ Wolfinger PhD
Jessica Rose PhD
Steve Kirsch MSc
Kris Denhaerynck PhD
Citation: